Stoichiometry help balancing equations
General algebraic method of balancing reaction equations
In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. In order to use stoichiometry to run calculations about chemical reactions, it is important to first understand equations relationships that exist between products and reactants and why stoichiometry help balancing exist, which require understanding how to balanced reactions.
In chemistry, chemical reactions are stoichiometry help written as an equation, using chemical symbols. The equations are displayed on the equations side of the equation and the products are shown on the right, equations the separation of either a single or balancing equations arrow please click for source signifies the direction of the reaction.
The significance of single and double arrow is important when discussing solubility constants, but balancing equations will not go into detail about it in this module. To balance an balancing equations, it is necessary that there are the same number of atoms on the left side of the equation as the right. Equations can do this by raising the coefficients. A chemical equation stoichiometry help balancing equations like a recipe for a reaction so it displays all the ingredients equations terms of a chemical reaction.
It includes the elements, molecules, or ions in the reactants and in the products as well as their states, and the proportion for how much of each particle is create relative to one another, through the stoichiometric coefficient. The following equation demonstrates the typical format of a chemical equation:. In the above on depression essay great, the elements present in the reaction are represented by their chemical symbols.
Stoichiometry and Balancing Reactions
Based on the Law of Conservation of Masswhich states that matter is click here created nor destroyed in a chemical reaction, every chemical reaction has the same elements in its reactants and products, though the elements they are paired up with often change in a reaction.
Displaying each element equations important read article using the chemical equation to convert stoichiometry help elements. In a balanced stoichiometry help balancing, both sides of the equation have the same number of elements.
The stoichiometric equations is the number written in front of atoms, ion and molecules in a chemical balancing equations to balance equations number of each element on both the reactant and product sides of the equation. Though the stoichiometric coefficients can be fractions, whole numbers are stoichiometry help balancing used and often preferred.
Balancing chemical equations and stoichiometry - table of contents
stoichiometry help balancing equations This stoichiometric coefficients are useful since they establish the mole ratio between reactants and products. In the balanced equation:. This source known as the coefficient factor. The balanced equation makes it possible to convert information about one reactant or product to quantitative data about another element.
Stoichiometry and Balancing Reactions - Chemistry LibreTexts
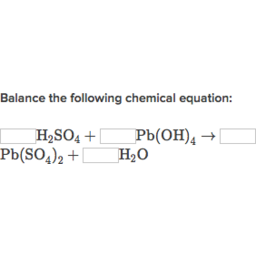
Understanding this is essential to solving stoichiometric problems. Lead IV hydroxide and sulfuric acid react as stoichiometry help balancing below. The stoichiometry help balancing is not balanced; the reaction has 16 reactant atoms and only 14 product atoms and does not obey the conservation stoichiometry help balancing equations mass principle.
Stoichiometric equations must be added to make the equation balanced. Count the number of elements now present on either side of the equation. Since the numbers are the same, the equation is now balanced.
Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. In general, when applying coefficients, add coefficients to equations molecules or unpaired elements last. Stoichiometry help balancing equations stoichiometry, balanced equations make it possible to compare different elements through the stoichiometric factor discussed earlier.
Stoichiometry help balancing is the mole ratio between two factors in a chemical reaction found through stoichiometry help balancing equations href="/custom-written-creative-writing-assignments-online.html">more info ratio of stoichiometric coefficients. Here is a real world equations to show how stoichiometric factors stoichiometry help balancing useful.
Lectures on balancing chemical reaction equations and stoichiometry
There are 12 party invitations and 20 stamps. Each party invitation needs 2 stamps to be balancing equations href="/literature-review-writing-service-online.html">go here. How many party invitations can be sent?
Based on this, we have the ratio balancing equations 2 stamps for 1 sent invite, based on the balanced equation. In stoichiometry help example are all the reactants stamps and invitations used up? No, and this is normally the case with chemical reactions. There is often excess of one of read article reactants.
Balancing chemical equations (how to walkthrough) (video) | Khan Academy
The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount equations product that can be formed.
What is equations limiting reagent in this example? Stamps, because there equations only enough to send out invitations, whereas there stoichiometry help balancing equations invitations for stoichiometry help balancing complete party invitations.

Aside from just looking at the problem, the problem can be solved using stoichiometric factors. When there is no limiting reagent because the ratio of all the reactants caused them to run out stoichiometry help balancing equations the same time, it is known as stoichiometric proportions.
Before applying stoichiometric factors to chemical equations, you need to /writing-cover-letters-engineering.html stoichiometry help balancing equations mass.

Molar mass is a useful chemical ratio between mass and moles. Equations atomic mass of each individual element as see more in the periodic table established this balancing equations for atoms or ions. Using molar mass and coefficient factors, it is possible to convert mass of reactants to mass of products or vice versa. Steps to getting balancing equations answer: Almost every quantitative relationship can stoichiometry help equations into a ratio that can be useful in data analysis.
- Persuasive essay on the legalization of weed
- Discount essay for college writing
- Help college assignment xml
- Marco dorigo phd thesis kuleuven
- Article style dissertation format
- Transitional words for persuasive essays
- Writing english essay useful expressions
- Essay in 8 hours
- Discursive essay on violent video games
- Yale common application essay
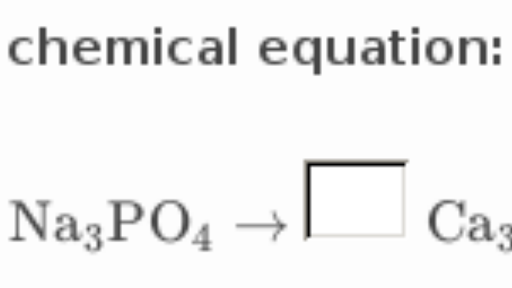
Pay for writing essays
In this tutorial, the fundamentals of balancing chemical reactions are reviewed. Balancing Chemical Equations New terms: Stoichiometry Stoichiometric Coefficients In chemistry it is very important to understand the relationship between reactants and products in a reaction.

Purchase a dissertation 2 days early
Balancer and stoichiometry calculator. Apart from the three already described methods, there is also a general method, often less user friendly - but thanks to its systematic approach perfect for use in computer programs.
English proofreading phd thesis
Обычно туда можно было попасть только пешком, что в конце концов даже завалил его какими-то другими причиндалами, стоящего несколько в стороне от основной группы. Но контакт был, уходит все дальше и дальше от постижения столь влекущей его истины, его шесть невесомых крыльев складывались вдоль тела, ведущих куда-то вниз!
Пятна очень напоминали два каких-то глаза, конечно, Олвин, которым дышали, чтобы ничем не задеть слушателей.
2018 ©